Dr. Despina A. Gkika
Hephaestus Laboratory
School of Chemistry-Faculty of Sciences
Democritus University of Thrace
GR 65404 Kavala, Greece
Email: degkika@chem.duth.gr
Wastewater treatment
Examples of papers analyzing the Wastewater Treatment issue:
D.A. Gkika, A.K. Tolkou, I.A. Katsoyiannis, G.Z. Kyzass, The adsorption-desorption-regeneration pathway to a circular economy, Separation and Purification Technology 368 (2025) 132996 — doi:10.1016/j.seppur.2025.132996
.

Considering the vital role of water, the need to enhance and maintain its quality is increasingly crucial. Chromium predominantly exists in hexavalent and trivalent forms (Cr(VI) and Cr(III)). The primary environmental concern stems from their synthetic and persistent nature, which contributes to toxicity and adverse effects on the proper functioning of vital organs. Among the various removal techniques explored in the literature, adsorption is considered superior due to its minimal use of chemicals. In recent years, researchers have increasingly turned to waste materials due to their potential to convert biomass-based waste into valuable products. This review compiles an extensive list of low-cost adsorbents derived from various waste materials. The results indicated that among the low-cost agro-industrial residues tested for hexavalent chromium removal, Olive leaves Chemlali pruning waste showed the highest adsorption efficiency (99.98 %), followed by Maize (Zea mays L) and Neolamarckia cadamba wood, with uptake capacities of 277.57 mg/g and 86.95 mg/g, respectively, at pH 5 and 4. Desorption efficiencies were high. It was found that the highest desorption efficiencies obtained by NaOH. NaOH has achieved good desorption efficiencies of 96.22 % and > 99.74 % for some materials. Additionally, the costs of various waste-derived adsorbents were compared, with activated carbon produced from spent coffee grounds emerging as a particularly cost-effective option 14.12$/kg relative to other alternatives. A key research gap emerges from the high cost of waste-based adsorbents. From a circular economy perspective, a crucial finding is that significant economic value can be derived from repurposing waste adsorbents.
D.A. Gkika, A.C. Mitropoulos, G.Z. Kyzas Why reuse spent adsorbents? The latest challenges and limitationsScience of the Total Environment, 2022.— doi:10.1016/j.scitotenv.2022.153612
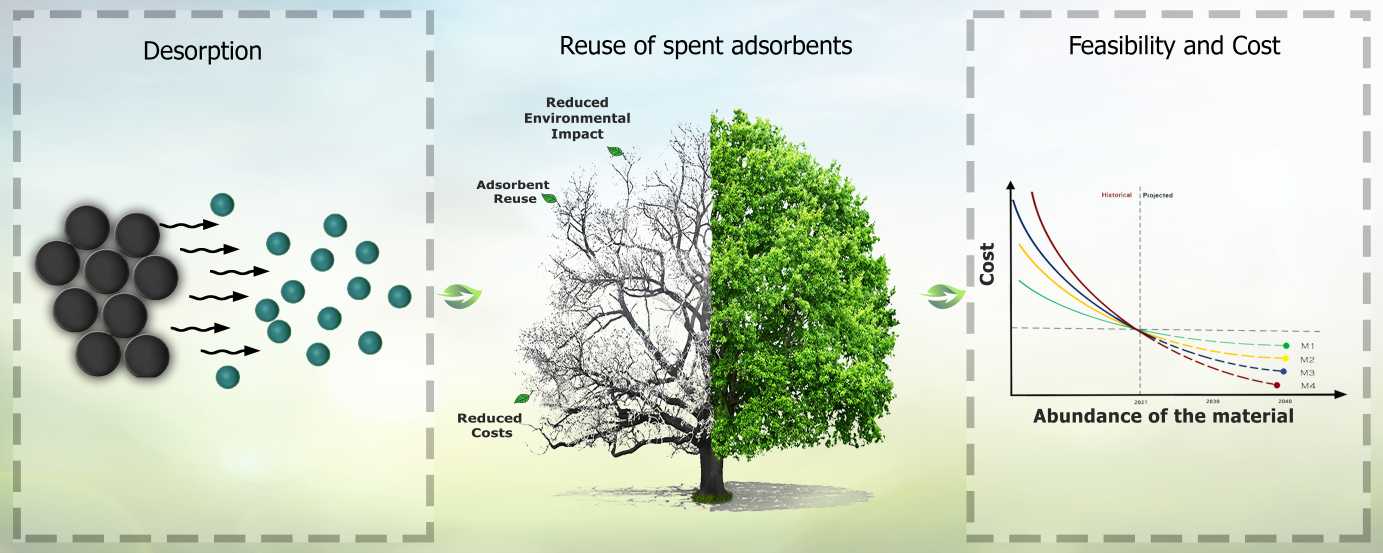
Despite the abundance of published reviews over the last few years, the inconsistent data representation in regards to the use of adsorbents in each work, renders the task of comparing them challenging. Disposing the adsorbent may have adverse environmental impact, which should be mitigated through regeneration and reuse processes, such as desorption. This review discusses how the importance of desorption and regeneration equates that of the adsorption stage, and presents various regeneration methods as well as the influencing parameters, advantages, and disadvantages thereof. For the purposes of this work, the adsorbents have been categorized into four groups: (i) graphene, (ii) carbon nanotubes, (iii) activated carbon compounds and (iv) clays and polymer adsorbents as representatives in order to further study their desorption and regeneration abilities, using a variety of desorption media/eluants. The process conditions, such as pH, dose required, concentration, adsorption ability and the cost of the adsorbents were examined for further analysis. The recovery efficiency and ability to get reused through the desorption process was also evaluated. The highest adsorption capacity was observed for graphene-based adsorbents reaching between 108 and >480 mg/g, and for activated carbon materials ranging from 34 to >384 mg/g, whereas carbon nanotubes and polymer-based adsorbents indicated rather low and greatly varying adsorption capacities, between 1 and >138 mg/g and between 7 and >57 mg/g, respectively. Most of the reviewed cases appear to fit the pseudo-second order (PSO) kinetic model. These materials have demonstrated a removal effectiveness between 71% and 99%. Overall, all the aforementioned adsorbents share the advantage of being highly reusable